Laparoscopic resection of left liver
segments using the intrahepatic
Glissonian approach
Machado MA, Makdissi FF, Surjan RC, Herman P, Teixeira AR, C Machado MC.
Surg Endosc. 2009; 23:2615-2619.

Machado MA, Makdissi FF, Surjan RC, Herman P, Teixeira AR, C Machado MC.
Surg Endosc. 2009; 23:2615-2619.
Laparoscopic resection of left liver segments using intrahepatic Glissonian approach
Marcel Autran Machado, F. F. Makdissi, R. C. Surjan, P.Herman, A. R. Teixeira, M. C. C Machado
Introduction
Recent advances in laparoscopic devices and experience with advanced techniques have increased the indications for laparoscopic liver resection [1,2]. Although laparoscopic liver resections are considered as technically demanding and potentially hazardous procedures, several authors have described growing number of procedures [2-5].
One of the main steps of liver resection is pedicle control. Anatomic hemihepatectomies require extensive hilar dissection with portal vein and hepatic artery control, whereas segmental resections are often performed without pedicle control or with selective Pringle maneuver [6].
The indications for laparoscopic segmental liver resections are increasing but most reported laparoscopic liver resections are hemihepatectomies or non-anatomical resection of liver segments. Intrahepatic Glissonian approach is useful for laparoscopic right hemihepatectomies and for segmental liver resection [7-9]. We have previously described a technique to perform resection of left liver segments, including left hepatectomy (resection of segments 2, 3 and 4), bisegmentectomy2-3 and anatomical resection of segments 2, 3 and 4, using small liver incisions according to anatomical landmarks such as the Arantius´ and round ligaments [10]. Using the same concept, this article describes a novel technique for laparoscopic resection of left liver segments using an intrahepatic Glissonian approach.
Patients and Methods
Nine consecutive patients underwent laparoscopic left liver resections using the intrahepatic Glissonian approach technique from April 2007 to June 2008. There were 7 women and two men with mean age of 41.9 years (range 27-64 years). Five patients had liver metastasis; three patients had hepatocellular adenoma with a mean size of 11.6 cm (range 7-19 cm) and one patient primary intrahepatic lithiasis. The surgical procedure, postoperative course and outpatient follow-up were evaluated and the following data collected prospectively: duration of surgery, average time to inflow pedicles control, perioperative transfusions, postoperative complications and hospital stay. The interval timing to control portal pedicles was defined by the time between beginning of intrahepatic dissection of Glissonian sheaths and establishment of ischemic delineation.
Operative Technique
The patient is placed in a supine position with the surgeon standing between patient’s legs. An orogastric tube is inserted and removed at the completion of the procedure. This technique uses five trocars. Using an open technique, a 12-mm trocar is placed 3 cm above the umbilicus; through this port, a 10-mm 30-degree angled laparoscope is introduced. Pneumoperitoneum is established at a pressure of 12 mmHg. The other four trocars are located as shown in Figure 1.

FIGURE 1. Diagrams of trocar placement for laparoscopic left liver resections.
Three 12mm trocars (red) and two 5mm trocars (blue) are used.
The round ligament is transected using laparoscopic coagulation shears (LCS; Ethicon Endo Surgery Industries, Cincinnati, OH, USA). Exploration of the abdominal cavity and ultrasound liver examination are performed.
Left liver is mobilized by sectioning the falciform, left triangular and coronary ligaments. Left lobe is pulled upward and the lesser omentum is divided exposing the Arantius’ ligament (ligamentum venosum). This ligament runs from the left branch of portal vein to the left hepatic vein or to the common trunk [11] being a useful anatomical landmark for the identification of left hepatic and portal veins. Arantius’ ligament is divided. The cephalad stump can be used as a landmark to dissect the left hepatic vein and the common trunk as described elsewhere [12]. The caudal stump of the ligament is used as a landmark for the left Glissonian pedicle (shown at A in Figure 2a). A small (3 mm) anterior incision is made in front of the hilum (shown at B in Figure 2a), and a large vascular clamp is introduced through the left side of the left Glissonian sheath behind the caudal stump of Arantius’ ligament toward anterior incision allowing the encircling of the left main sheath (Figure 2b) that can be easily divided with an endoscopic vascular stapler during a left hemihepatectomy (Figure 3).

FIGURE 2. Schematic view of intrahepatic Glissonian access for laparoscopic left liver resections.
a. Landmarks for access of the left liver segments Glissonian pedicles. Site A indicates the caudal stump of the Arantius ligament; B, the anterior incision in front of the hilum; C, the basis of the round ligament, right side; D, the basis of the round ligament, left side; E, midway between sites D and A.
b. Left hemihepatectomy - A large laparoscopic vascular clamp is introduced through incisions A and B to occlude left Glissonian pedicle.
c. Bisegmentectomy 2-3 – Combining incisions A and D it is possible to occlude the Glissonian pedicle of segments 2 and 3.
d. Segmentectomy 4 – Combining incisions B and C and using the same maneuver it is possible to occlude the Glissonian pedicle of segment 4.
e. Segmentectomy 2 – Combining incisions A and E and using the same maneuver it is possible to occlude the Glissonian pedicle of segment 2.
f. Segmentectomy 3 – Combining incisions E and D and using the same maneuver it is possible to occlude the Glissonian pedicle of segment 3.

FIGURE 3. Laparoscopic left hemihepatectomy (resection of segments 2,3 and 4).
a. Intraoperative view of ischemic delineation of the left liver. Note vascular endoscopic stapler encircling left Glissonian pedicle.
b. Schematic view – Stapler is closed and ischemic delineation of left liver is obtained
c. Intraoperative view. Stapler is fired and left main Glissonian pedicle is transected (arrows).
d. Schematic view – Stapler is fired.
This maneuver spares the caudate lobe (segment 1) portal branches. The round ligament is then retracted upward exposing the umbilical fissure between segments 3 and 4. In about one third of the patients a parenchymal bridge connecting these two segments is present and must be divided. Using the round ligament as a guide, two small incisions (C and D in Figure 2a) are performed on the left and right margins of the round ligament where it is possible to identify the anterior aspect of Glissonian pedicle of segment 4 on its right side and segment 3 on its left side. By combining the incisions A and D (Figure 2c), it is possible to control the Glissonian pedicle of the left lateral sector (segments 2 and 3). With a clamp introduced through incisions B and C, it is possible to reach Glissonian pedicle of segment 4 (Figure 2d). Another small incision can be performed in the midway between incisions A and D (E in Figure 2a) permitting individual access either to segment 2 or 3 (Figures 2e and 2f), allowing individual resection of segments 2 and 3 (Figure 4). All this steps are performed without the Pringle maneuver and without hand assistance. Left hepatic vein (Figure 5) or the common trunk can be dissected and encircled following anatomical landmarks described elsewhere [11,12].
Parenchymal transection and vascular control of the hepatic veins are accomplished with harmonic scalpel and endoscopic stapling device as appropriate. The specimen is extracted through a suprapubic incision, inside a plastic bag. One round 19F Blake abdominal drain (Ethicon, Inc, Cincinnati, Ohio) was left in place in all patients.
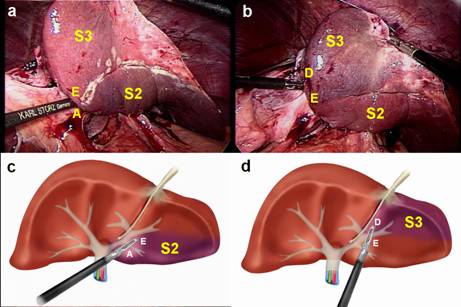
FIGURE 4. Laparoscopic anatomical resection of segments 2 and 3.
a. Intraoperative photograph. Glisson pedicle is occluded and ischemic delineation of segment 2 (S2) is obtained. Segment 3 is not ischemic (S3).
b. Intraoperative photograph. Glisson pedicle is occluded and ischemic delineation of segment 3 (S3) is obtained. Segment 2 is not ischemic (S2).
c. Schematic view – A large laparoscopic vascular clamp is introduced through incisions A and E resulting in ischemic delineation of segment 2 (S2).
d. Schematic view – A large laparoscopic vascular clamp is introduced through incisions E and D resulting in ischemic delineation of segment 3 (S3).

FIGURE 5. Laparoscopic dissection of left hepatic vein. Intraoperative view.
a. Arantius’ ligament (AL) is divided.
b. Dissection between middle (MHV) and left hepatic vein (LHV) is accomplished with laparoscopic right angle dissector.
c. The cephalad stump of Arantius’ ligament (AL) can be used as a landmark to dissect the left hepatic vein (LHV).
d. Left hepatic vein (LHV) is encircled by right angle dissector. Middle hepatic vein (MHV) is not included in the dissection.
Results
Five patients underwent laparoscopic bisegmentectomy 2-3, one laparoscopic left hemihepatectomy (Figure 3), two resection of segment 3 and one resection of segment 4. Blood transfusion was required in one patient. Mean time consumed to achieve complete control of pedicles was 4.7 minutes (range 2-16 minutes) and mean operative time was 180 minutes (range 120-300 minutes). The median hospital stay was 3 days (range 1-5 days). No patient had postoperative signs of liver failure or bile leakage. No postoperative mortality was observed.
Four patients were operated on for benign primary tumor while the remaining five were operated on due to malignant secondary tumors. These five patients had negative surgical margin, and were > 1cm in all patients. One patient exhibited moderate post chemotherapy steatohepatitis and two had mild steatosis. Intraoperative laparoscopic liver ultrasound confirmed the site and size of the lesions diagnosed by CT scan and/or MRI.
Discussion
The knowledge of segmental liver anatomy, as described by Couinaud has provided the basis for segmental liver resections. Liver can be divided into eight different segments: segment 1 as the caudate lobe; segments 2, 3 and 4 as left liver and segments 5, 6, 7 and 8 as the right liver.
The main indication for segmental approach is to preserve liver parenchyma, especially in cases with bilateral lesions or cirrhotic livers. It permits complete anatomical clearance of the disease, leaving adequate functioning liver, by removing individual hepatic segments.
Removal of anatomical segments of the liver by laparoscopy is a difficult task. Indeed, most performed laparoscopic liver procedures are right or left hemihepatectomies, left lateral segmentectomy and nonanatomical liver resections [3]. Anatomical segmental liver resection is not currently performed due to technical difficulties to control segmental Glissonian pedicles laparoscopically. The authors have previously reported a technique for laparoscopic right segmental liver resections [10] and based on this experience, describe a systematized technique to reach the left Glissonian pedicles and remove any left liver segments by laparoscopy. These techniques permit a tailored liver resection by removing only the liver segments involved by the underlying disease. Respect of anatomic landmarks of liver segments during resection prevents impairment of the vascularization of the remaining parenchyma and excessive bleeding.
Pringle maneuver is a main step during laparoscopic liver resection and it is used to avoid major bleeding but can be associated with prolonged ischemic time. Laurent et al.[13] showed that laparoscopic liver resections are associated with higher ischemic time. The present technique precludes the use of Pringle maneuver and permits not only hemihepatectomy but also segmental liver resections.
Outflow control is of utmost importance during liver resection and it was possible to be accomplished whenever necessary (Figure 5). Anatomic landmarks described elsewhere can be used to guide the laparoscopic surgeon to a safe dissection of both left and middle hepatic veins (Figure 5).
This novel technique can expand the indication for laparoscopic segment-based liver resections, sparing liver parenchyma. Preservation of liver parenchyma should always be attempted in order to prevent postoperative liver failure and to increase the opportunity to perform repeated resections in cases of recurrent malignancy [14].
Left hemihepatectomy can be achieved with dissection of the left hepatic artery, duct, and portal vein separately [2-5] which is tedious, time-consuming and may jeopardize vascular and biliary vessels if anatomical variation is present. The present technique, based on small incisions following specific anatomical landmarks, allows a straight forward control of Glissonian pedicle (Figure 3) without hilar or parenchymal dissection and without ultrasound or cholangiography guidance. This technique precludes encircling of the Glissonian pedicles thus simplifying the procedure and minimizing bleeding from this blunt maneuver which is much more difficult to be done laparoscopically.
The main advantage over other techniques is the possibility to gain a rapid and precise access to every Glissonian sheath of segments 2, 3 and 4 facilitating left hemihepatectomy, bisegmentectomy 2-3, and resection of liver segments 2, 3 and 4.
We believe that the described technique facilitates laparoscopic liver resection by reducing the technical difficulties in pedicle control and may increase the development of segment-based laparoscopic liver resections.
Acknowledgments
We appreciate the continued support from the Sirio Libanês Hospital and Dr Paulo
Chapchap. We are also grateful to Mrs Valéria Lira da Fonseca for the drawings.
References
1. Koffron A, Geller D, Gamblin TC, Abecassis M. Laparoscopic liver surgery: Shifting the management of liver tumors. Hepatology 2006; 44:1694-700.
2. Dagher I, Proske JM, Carloni A, Richa H, Tranchart H, Franco D. Laparoscopic liver resection: results for 70 patients. Surg Endosc 2007; 21:619-24.
3. Gagner M, Rogula T, Selzer D. Laparoscopic liver resection: benefits and controversies. Surg Clin North Am 2004; 84:451-62.
4. Vibert E, Perniceni T, Levard H, Denet C, Shahri NK, Gayet B. Laparoscopic liver resection. Br J Surg 2006; 93:67-72.
5. Koffron AJ, Auffenberg G, Kung R, Abecassis M. Evaluation of 300 minimally invasive liver resections at a single institution: less is more. Ann Surg 2007; 246:385- 92.
6. Machado MA, Makdissi FF, Bacchella T, Machado MC. Hemihepatic ischemia for laparoscopic liver resection. Surg Laparosc Endosc Percutan Tech 2005; 15:180-3.
7. Topal B, Aerts R, Penninckx F. Laparoscopic intrahepatic Glissonian approach for right hepatectomy is safe, simple, and reproducible. Surg Endosc 2007; 21:2111.
8. Cho A, Asano T, Yamamoto H, Nagata M, Takiguchi N, Kainuma O, Souda H, Gunji H, Miyazaki A, Nojima H, Ikeda A, Matsumoto I, Ryu M, Makino H, Okazumi S. Laparoscopy-assisted hepatic lobectomy using hilar Glissonean pedicle transection. Surg Endosc 2007; 21:1466-8.
9. Machado MA, Makdissi FF, Galvão FH, Machado MC. Intrahepatic Glissonian approach for laparoscopic right segmental liver resections. Am J Surg 2008; 196:e38-42.
10. Machado MA, Herman P, Machado MC. Anatomical resection of left liver segments. Arch Surg 2004; 139:1346-9.
11. Ferraz de Carvalho CA; Rodrigues AJ Jr. Contribution to the study of functional architecture of the ligamentum venosum in adult man. Anat Anz 1975; 138:78-87.
12. Majno PE; Mentha G; Morel P et al. Arantius’ligament approach to the left hepatic vein and to the common trunk. J Am Coll Surg 2002;195:737-39.
13. Laurent A, Cherqui D, Lesurtel M, Brunetti F, Tayar C, Fagniez PL. Laparoscopic liver resection for subcapsular hepatocellular carcinoma complicating chronic liver disease. Arch Surg 2003; 138:763-9.
14. Nishio H, Hamady ZZ, Malik HZ, Fenwick S, Rajendra Prasad K, Toogood GJ, Lodge JP. Outcome following repeat liver resection for colorectal liver metastases. Eur J Surg Oncol 2007; 33:729-34.